Atomic structure
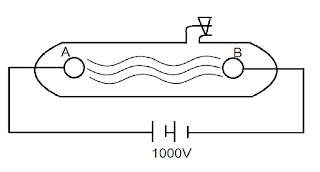
Discharge tube or crook's tube
Discharge tube :-
• Discharge tube is a long glass tube closed at both ends to circular metal
plates A and B are sealed at two ends of the tube. These plate are called
electrode.
• Discharge tube which can be connected to vacuum pump to a struck out their
or gas present inside the tube.
• The two plates can be connected to a source of very high voltage.
• The plate which is connected to tu negative source called cathode.
• The plate which is connected positive source called anode.
Experiment and observation :-
• When the pressure of gas in the discharge tube is at 1 atm and high voltage
applied across the electrode, no current flows through the air across the
electrode.
• When the pressure of gas is lower about 10 mm of Hg the irregular strikes of
light becomes a continuous column of light that glows. the columna starts from
anode and reaches almost up to the cathode this column is called the positive
column.
• As the pressure of the gas reduces further the length of positive column
reduces. it now ends well before cathode find glow can be see at cathode also,
the dark space left between positive column and cathode glows is called
faraday's dark space.
• When the pressure of gas is reduced to about 1 mm of Hg cathode glow moves
away from the cathode, creating a dark space between cathode and cathode glow
this space is known as crooke's dark space.
• At a pressure of 0.1 mm of Hg the positive column gets splits into number of
band called Steriation. As the pressure is reduced the steriation moves
towards the anode and finally vanished at about 0.01 mm of pressure.
• At the pressure 0.01 mm of Hg the space between the electrodes filled with
crooke's dark space.
• At this stage (0.01 mm of Hg) the glass tube begins to glow mainly at the
end of opposite to the cathode this phenomenon is called fluorescence.
• The colour of the glow depends upon the nature of glass used and the type of
fluorescence used at the end of the glass tube.
• These invisible rays starting from the cathode so these are called cathode
rays.
• The nature of cathode rays is independent of the nature of gas through which
the electric discharge takes place.
Properties of cathode rays :-
• Cathode rays travels in a straight line.
Explanation :- if an object such as a metal is placed in the path of cathode
rays they cast a sharp shadow of the object at the back.
• Cathode rays are made up of material particles.
Explanation :- if light paddle wheel is placed in the path of cathode rays
such that cathode rays strikes the Blade of upper half it begins to rotate.
• Cathode rays carries negative charges.
Explanation :- When an electric field is applied on the cathode rays they are
deflected towards the positive plate of the electric field.
when a magnetic field is applied on the cathode rays they are deflected in the
direction anode which shows that they carry negative charges.
• They produce green fluorescence on the glass walls of the discharge tube as
well as on certain substances such as zinc sulfide.
• Cathode rays produce heating effect.
• They produce X-rays strikes against the surface of hard metal like tungsten
or malayidum.
• The two most important observe from above observations :-
1. Cathode rays are made up of material particles.
2. Cathode rays carry negative charges.
Electron
The negatively charged material particle constituting cathode ray.
Charge and mass of the electron :-
For determination of ratio e/m of the electrons.
• JJ Thomson in 1897 studied the event of deflection of cathode rays under the
influence of electric field and magnetic field of different strength. from
these result he determined the ratio of charge by mass of the particles
constituting the cathode rays.
Oil-drop experiment
- by R.A millikan(1917)
A spray of oil-drop is produced by an atomizer the oil-drop enter the
operators through a small and are allowed to fall in between two charged
plates the space between the two charged plates is ionized by X rays which
ionized the molecules of air. The movement of droplet is observed with a
telescope. the droplets observe one or more electrons that mean acquire a
negative charge by applying the electric field on the droplet under study
which balance the downward gravitational force the droplet began stationery.
Mass of a hydrogen atoms = 1.008 amu
1 amu = 1.67 ✖ 10-24
g
comparison between mass of hydrogen q mass of an electron
mass of a hydrogen atom = 1837 ✖ mass of an electron
Origin of cathode rays :-
The cathode rays are first produced from the material of the cathode this then
hit the gas atoms present in the discharge tube and a knock-out electrons from
the gas atom these electrons travel towards the positively charged anode in
the form of cathode rays.
Discovery of proton :-
Study of anode rays and canal rays
Production of anode rays :-
Since the atom as a whole is electrically neutral and the presence of
negatively charged electron in it therefore it was throughout the some
positively charged particle must also be present in the atom.
• The existence of positively charged particles in atom was shown by Gold stin
in 1886
• On applying high voltage between the anode and the cathode, in this
experiment some rays are coming from the side of the anode which passed
through the hole in the cathode.
• The rays which are coming from side of anode therefore they are called
anode rays.
• They are also named as canal rays because they passed through the holes or
canal in the cathode.
Origin of anode rays :-
Anode rays don't originate from the anode they are produced in the space
between the anode and the cathode.
• It is believed that the high electrical energy is supplied between the
electrodes splits the molecule of gas present in the tube into atoms.
• The electrons present in these atoms further absorb electrical energy and
are knocked out.
• The electrons thus, knocked out, travel toward anode and form a part
of cathode rays.
• The remaining part of the atom becomes positively charged particles. this
positively charged particles travel in the form of a string towards the
cathode and constituted anode rays.
Characteristics of anode rays :-
• The e/m ratio for these rays is smaller than that of electrons.
• Unlike cathode rays, there e/m value is depend upon the nature of gas
taken in the tube for different gases used in the discharge tube.
• When hydrogen gas is taken in the discharge tube, the e/m value obtain for
the positive rays is found to be maximum, since the value of charge on the
positive particles obtained from the different gases is the same. The value
of "m" must be minimum for the positive particles obtained from hydrogen
gas.
• They are capable to produce ionization in gases.
• They can produce physical and chemical changes.
• They travels in a straight line.
• They are made up of material particles.
• They carry positive charge.
• Mass of the positively charged particles constituting the anode rays also
depend on the nature of gas.
• Charge on the positively charged particles constituting the anode rays
also depend on the nature of gas and voltage applied.
Proton
A proton is defined as that such atomic or fundamental particle which
carries one unit positive charge and has mass nearly equal to that of
hydrogen atom.
Atomic models
Thomson atomic model :-
• An atom is electrically neutral. It contains positive charge (due to the
presence of proton) as well as negative charge (due to the persons of
electron) hence JJ Thomson assumed that an atom is uniform a sphere positive
charge with electron embedded on it.
• Thomson atomic model is also known as plum pudding model.
• This model is often called watermelon model because the red part of the
watermelon is considered as uniform positive masses and seeds of watermelon
are electrons embedded on it.
Rutherford's Alpha scattering experiment :-
Observation
• Most of the Alpha particles passed straight through gold file without
suffering any deflection from their original path.
• A few of them where deflected through a small angles while a very few
where deflected to larger angle.
• A very small percentage(1 in 20000) was deflected through angle ranging
from 90⁰ to 180⁰.
Rutherford's nucleus concept of atom :-
• Most of the sphere in the atom is empty.
• The volume of the nucleus is negligible as compared to the volume of atom.
Radius of atom (R) =
10-10
Radius of nucleus (r) =10-15
R/r = 10-10 / 10-15
R = 105r
• The electrons are distributed in the empty space of the atom around the
nucleus in different concentric circular path called orbit.
• The atom of an element consists of a small positively charge nucleus which
is situated at the centre of the atom and which carries atoms most of the
entire mass of the atom.
• The number of electrons in orbits is equal to the number of positive
charge in the nucleus. hence, atom is electrically neutral.
Drawback of rutherford's model :-
• This was not according to the classical theory of electromagnetism
proposed by James clerk Maxwell.
According to this theory every accelerated charged particle must emit
radiation in the form of electromagnetic wave and losers it's total energy.
Since energy of electrons keeps on decreasing so radius of the circular
orbit should also decreases and ultimately the electrons should fall in
nucleus.
On the basis of Alpha scattering experiment, Rutherford forward a model of
atoms known as Rutherford's nuclear model of atom.
This model consists of two parts:-
1. Nucleus :- Nuclear is a small having positively charge body present in
the centre of atom.
2. Extra nucleus part :-
• The space around the nucleus in which the electron are distributed.
• The entire mass of the atom is concentrated in nucleus since the electron
have negligible mass.
• The mass of the atom is mainly due to proton and nucleus contain proton
and neutron.
Nucleus + proton + neutron = nucleon
• The presence of positively charged proton in the nucleus also account for
the positive charge in the nucleon.
To explain that the electron do not fall into the nucleus as a result of
attraction,
Rutherford suggested that electrons where a stationary but revolving around
the nucleus in certain circular orbits as a result centrifugal force comes
into plan which balance the force of attraction.
thus this model is similar to our solar system where the nucleus is like the
sun and electrons are like the planet that is why these electrons are also
caused planetary electrons.
Quantum theory of radiation :-
Energy can be absorbed or radiated by a body in the form of small packets of
energy called Quanta
• Which are whole number multiple of quantity.
E = h𝛎
𝛎 = 6.625 ✖ 10-34 j/sec
E = energy
h = Plank's constant
𝛎 = frequency
Bhor's models of atom :-
• Electrons revolve around the nucleus in a specified circular path called
orbit or shell.
• Each orbits or shell associated with a definite amount of energy hence
these are also called energy level and are designated K,L,M,N.
• The energy associated with a certain energy level increases with the
increase in its distance from the nucleus hence if the energy associated
K,L,M,N shell are e1, e2, e3, e4
respectively then.
e1 < e2 < e3 < e4
< .....
An atom of each element has definite combining capacity of the element is
called valency.
• The number of electrons gained, loosed or shared by the atom of an element
to complete octet stable.
Valence electron :- the electrons present in the outermost shell are valence
shell is called valence electron.
Atomic number :- the total number of protons present in the nucleus of an
atom is called atomic number.
• It is denoted by Z
Z = number of proton
For neutral atom :-
Z = number of proton
Z = number of proton =
number of electron
For positively charged atom (cation) :-
Z = number of proton >
number of electron
For negatively charged atom (anion) :-
Z = number of proton < number
of electron
Mass number :- the sum of the total number of protons and neutrons present
in the nucleus of an atom is called mass number.
• It is denoted by A
A = number of proton + number of neutron
A = atomic number + number of neutron
A = Z + n
n = A - Z
Symbolic representation of an atom
AZX or ZXA
Isotopes :- Isotopes are atoms of the same element of the same atomic number
but different mass number.
E.g. - Hydrogen 16C12 , 6C14
| Protium 1H1 | Duterium 1H2 | Tritium 1H3 | |
| Z | 1 | 1 | 1 |
| A | 1 | 2 | 3 |
| no. of neutrons = A - Z | 0 | 1 | 2 |
| no. of proton | 1 | 1 | 1 |
| no. of electron | 1 | 1 | 1 |
Application of isotopes :-
• Uranium (92U838) is used as fuel in nuclear
reactors.
• Cobalt (27Co60) is used in the treatment of
cancer.
• iodine (53I128) is used in the treatment of goitre
• sodium (11Na42) is used to differentiating cancer
tissue or normal tissue.
• carbon (6C14) is used to dating of fossil sample.
Isobar :- Atoms of different elements with different atomic number which
have the same mass number is called isobars.
E.g.- 18Ar40 , 20Ca40
Isotones :- Atoms having same number of neutrons are called isotones.
Isoelectronic :- Species having same number of electrons are known as ice
electronics.
E.g.-
18Ar , 19K+
,
20Ca2+ , 17Cl-
no of electrons : 18 , 19 - 1 = 18. 20 - 2 = 18. 17 + 1 = 18






















No comments:
Post a Comment