Atoms and molecules
• The smallest unit of any element is called an atom.
• The terms of different elements combine with one another to form New
substance called compound.
• The compound so formed are neutral in character it means they have no
electrical charge on them.
• All matter is made by the combination of atoms of different elements
combined together in some fixed ratio.
• Atoms are the basic building blocks of the matter.
Law of chemical combination :-
the chemical reactions which take place according to the certain laws called
the laws of chemical combination.
1.Law of conservation of mass
2.Law of constant proportions
3.Law of multiple proportions
4.Law of reciprocal proportions
5.Law of combining volume (gay lussac law of gaseous volume)
1. Laws of conservation of mass :-
• This law was first stated by lavoisier in 1774.
In all the chemical changes the total mass of the system remain constant.
Or
In a chemical change mass is neither created nor destroyed.
A + B ______ C + D
Total mass of (A + B) = total mass of (C + D)
Total mass of reactant = total mass of product.
2. Law of definite or constant proportion :-
• This law was presented by Proust in 1799.
a chemical compound always contains the same elements combined together in
fixed proportion by mass.
3. Law of multiple proportion :-
• This logon as put forward by Dalton in 1808
if two element combine to form more than one compound then the different
masses of one element which combined with a fixed mass of the other element,
bear a simple ratio to one another.
N2 + O2
| N | O | ||
| N2 O2 | 28 parts | 16 parts | 16:32:48:64:80 |
| N2 O2 | 28 parts | 32 parts | 1:2:3:4:5 |
| N2 O2 | 28 parts | 48 parts | |
| N2 O2 | 28 parts | 64 parts | |
| N2 O2 | 28 parts | 80 parts |
4. Law of reciprocal proportion :-
• This law was given by Ritcher in 1792
When definite mass of element A combines with two other elements B and C to
form two compounds and B and C also combined to form a compound, bear
combining masses are in the same proportion for their a simple ratio to the
masses of B and C which combined with the constant mass A.
5 law of gaseous volume :-
• This law was given by gay lussac
whenever gases react together the volumes of the reactive gases as well as the
products gases, bear a simple whole number ratio provided all the
volumes are measured under similar conditions of temperature and pressure.
E.g. - H2 (g) + Cl2 (g)
→ 2HCl (g)
1 : 1 : 2
2H2 (g) + O2 (g) → 2H2O (g)
2 : 1 : 2
Dalton's atomic theory :-
• John Dalton in 1808 put forward a theory is known as Dalton's atomic theory.
1. Each element is composed of extremely small particles called atoms.
2. all atoms of a given element are identical. the atoms of different elements
are different.
3. Atoms are neither created nor destroyed in a chemical reaction.
4. Compounds are formed when atoms of more than one elements combined, a given
compound always has the same relative number and kind of atom.
5. When atoms combine in with one another to form compound atoms (molecules),
they do so in simple whole number ratio such as
1:1, 1:2, 2:1 etc.
6. atoms of two element may combine in different ratio to form more than one
compound.
E.g. - C : O (CO2 = 1 : 2, CO = 1 : 1), S : O (SO2 = 1 :
2, SO3 = 1 : 3)
7. An atom is the smallest particle that takes part in a chemical reaction.
Drawback of Dalton's atomic theory :-
• According to Dalton's atomic theory, atoms where throughout to be
indivisible but atoms can be further divided into a smaller particles called
electron, proton and neutron.
• Dalton's atomic theory said that all the atoms of an element have exactly
the same mass but atoms of same element can have different mass.
E.g. - Isotopes 6C12 6C14 , 8O16 8O17 8O18
• Dalton's atomic theory said that atoms of different elements have
different masses but if it is known that even atoms of different elements
can have the same mass.
E.g. - 40k 40Ca
• it could explain the laws of chemical combination by mass but failed to
explain the law of gaseous volume.
Avogadro's law :-
equal volumes of all gases under similar condition of temperature and
pressure contain equal number of molecules.
Application of avogadro's law :-
• This law helped to remove anomaly between Dalton's atomic theory and gay
lussac's law of the volume by making a clear distinction between atoms
and molecules.
• it reveals that common elementary gases like hydrogen nitrogen Oxygen etc
are diatomic.
• it provides a method to determined the molecular weight of gaseous
elements.
Vapour
density :-
Molecular mass of a gas at STP (similar temperature and pressure) = 2*
V.D
Atoms :-
an atom is the smallest particle of an element which can take part in
chemical reaction.
• atoms of most of the elements have no free existence. however, they exist
in the combined state as molecules and ions.
• the atom of a noble gas only have free existence.
• each atom of an element shows all the properties of the element.
Size of an atom :-
• The diameter of an atom is about 10-10m
• the structure and property of individual atom is seen by scanning electron
microscope and electron tunneling microscope, which can magnify the image of
an object by millions time.
Symbols of atoms and elements :-
A symbol is an abbreviation for the full name of the element.
• JJ Berzelius who devised the modern system of representing element.
Rules which is used to represent a symbol :-
1. the abbreviation used to represent an element is generally the first
letter in capital of the English name of the element.
Example :- Hydrogen = H
Oxygen = O
Fluorine = F
Sulphur = S
Phosphorus = P
2 . when the names of two or more elements begins with the same initial
letter. the initial letter followed by one of the next letters is used to
symbolise the element.
Example :-. Barium = Ba
Bismuth = Bi
Bromine = Br
Calcium = Ca
Cadmium = Cd
Chromium = Cr
3. The symbols of some elements are derived from their Latin name.
Sodium – Natrium (Na)
Potassium – Kalium (K)
Iron – Ferrum (Fe)
Copper – Cuprum (Cu)
Silver – Argentum (Ag)
Tin – Stannum (Sn)
Antimony – Stibium (Sb)
Tungsten – Wolfram (W)
Gold – Aurum (Au)
Mercury – Hydrargyrum (Hg)
Lead – Plumbum
Importance of symbol :-
• It represents one particular element.
• it represents one atom of the element.
• it represents one atomic mass of the element.
Molecule :-
The smallest particles of matter (element, compound) which can exist in a
free state is called a molecule.
Properties of molecule :-
• The properties of a substance are the properties of it's molecule.
• the molecules of different substances are different.
Compound molecule :- the molecule of a compound contains two or more atoms
of different elements.
Example :- H2O (contains one atom of Oxygen and two atom of hydrogen)
Atomicity :- the number of atoms contained in the molecule of a substance is
called atomicity.
Types of molecules :-
1. Monoatomic molecules :- one atom/ molecule.
Example :- noble gases (He, Ne, Ar, Xe, Kr, Rn)
2. Diatomic molecules :- these molecules consists of two atoms.
Example :- Element = H2,
Cl2, N2, O2 etc.
Compound = CO, NaCl, SO etc.
3. Triatomic molecules :- these molecules consist of three atoms.
Example :-. Element = O3
Compound = CO2, H2O, H2S, NaOH.
4. Tetra atomic molecule :- these molecules consist of four atoms.
Example :- P4
5. Polyatomic molecules :- any molecule can be containing more than four
atoms are known as polyatomic molecule.
Example :- Na2CO3, CH3OH, H2SO4,
HNO3, C2H5OH etc.
Ions :- an atom or group of atoms which contain charge (either positive or
negative) are called iron.
• Positive ions are called cations.
Example :- Mg2+ , Al3+ , Na+ , K+
• negative ions are called anions.
Example :- SO42- , O2- , NO3- , CO32- , Cl-
Valency :- the valency of an element is defined as its capacity to combine
with other element is known as valency.
Monovalent :- do jal mein aansu whose valency is 1.
Example :- H, Cl, Br, I, Na, K
Divalent :- whose valency is 2
Example :- O, S, Ca, Zn, Mg
Trivalent :- whose valency is 3
Example :- Al, B, N, Ag
Tetravalent :- whose valency is 4
Example :- C, Si, S
Variable valency :- there are some element which show different valency in
different compound.
| Elements | Lower valency | Higher valency |
| Cu | 1 | 2 |
| Fe | 2 | 3 |
| Ag | 1 | 2 |
• The compound in which the element shows the higher valency is indicated by
the suffix - ic.
• the compound in which the element shows the lower valency is indicated by
the suffix - ous .
Example :-. Ferrous oxide = Fe+2 O2-
Ferric oxide = Fe2+3 O32-
Molecular or chemical formula :-
A molecular formula is the representation of a chemical compound using a set
of symbols for the atoms of element present in a molecule of the compound,
and shows the actual number of atoms of each element of the compound.
Empirical or simple formula :-
the empirical formula of a compound gives the simplest whole number ratio of
the atom of the element present in the molecules of the compound.
Molecular formula of Glucose = C6H12O6
Molecular formula = n ✖ empirical formula
n = molecular mass of the compound/empirical formula
molecular mass = 180 Empirical mass = CH2O
12 + 2 ✖ 1 + 16 = 30
n = 180/30 = 6
Molecular formula = 6(CH2O)
= C6H12O6
Steps taken to arrive at the empirical formula :-
1. Determine the percentage of each element.
2. divide the percentage of each element by it's atomic mass to obtained the
relative number of atom (atomic ratio).
Atomic ratio = % of an element / atomic mass of the same elements
3. Divide the atomic ratio by simplest Quotient to get the simplest ratio of
various elements.
4. Convert the simplest ratio to whole number ratio.
(a) rounding off
E.g. - 1.98 ≈ 2
1.64 ≈ 2
2.34 ≈ 2
(b) multiply by integer
E.g. - 1.25 ⇒ 1.25 ✖ 4 = 5
1.5 ⇒ 1.5 ✖ 2 = 3
Mole concept
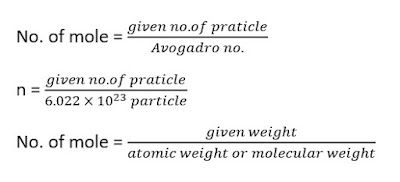
1 mole :- one mole of any substance is that quantity in which the chemical
species present are same in the number as the number of atom in the 12 gram
of pure carbon - 12 (C-12)
The number of atoms in 12 gram of C-12 = 6.022 ✖ 1023 atm
1 mole = 6.022 ✖ 1023
piratical
• In 1 gram atomic weight of any element 6.022 ✖ 1023 atm of
that element are present in this quantity of element is known as one mole.
Example :-
1 mole of atomic nitrogen (N-14) = 6.022 ✖ 1023 atm = 14
gram of N
1 mole of molecular nitrogen (N2) = 6.022 ✖
1023 molecule = 28 gram of N2
1 mole of water (H2O) = 6.022 ✖ 1023 molecule =
18 gram of H2O
1 mole of sulphate ion (sO42-) = 6.022 ✖
1023 ion = 96 gram of ___ ions
1 mole of NaCl ion (NaCl) = 6.022 ✖ 1023 molecule = 58.5
gram of NaCl
Atomic mass
Atomic mass of any element is the number of times an atom of that element is
heavier than an atom of carbon takes as 12.
Atomic mass = mass of n atom of an element / mass of an atom of C-12
Atomic mass unit (amu) = 1/12 of mass of an atom of C-12
1 mole of C-12 contains NA particles of mass = 12 gram
1 particles of mass = 1 /NA = 1 / 6.022 ✖
1023 = 6.022 ✖ 10-24
Average atomic mass :-
= Σ (% abundance ✖ isotopic mass) / 100
= Σ (isotopic mass ^ fractional abundance)
Ions :-
The electrically charged particles are formed when electron are added to or
removed from neutral atom are called ions.
• Cation :- when electron is removed from the valenced shell of an atom,
then cation is formed.
Example :- Na + energy → Na+ +e-
Mg +
energy → Mg2+ +2e-
Al +
energy → Al3+ + 3e-
• Anion :- when electron is added to the valence shell of an atom, then
cation is formed.
Example :- O + 2e- → O2- + energy
Cl + e- → Cl- + energy
P + 3e- → P3- + energy
Calculation of moles of atoms in a compound :-
Compound = AxBy
Number of moles of A = x ✖ number of moles of AxBy
Number of moles of B = y ✖ number of moles of AxBy








No comments:
Post a Comment